Answer:
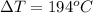
Step-by-step explanation:
Hello,
In this case, the relationship between the mass, heat, temperature and heat capacity id given by:
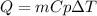
In such a way, the temperature change results:
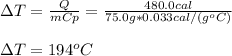
Clearly it is a positive change which means the temperature increases as heat is added.
Regards.