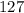Step-by-step explanation:
Using the Combined Gas Law, which is:
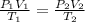
(With
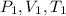 being initial pressure, volume and temperature; and
being initial pressure, volume and temperature; and
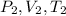 being the new values)
being the new values)
We can move the units around in order to solve for
 , which would look like this:
, which would look like this:
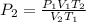
Then we convert the Celsius temperature to Kelvin:
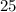 °
°
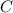 =
=
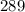
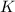
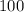 °
°
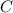 =
=
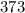
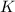
And now, we plug in all of the values and solve, with volume remaining as a constant:
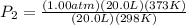

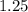
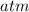 or
or