Answer:
The bracelet is made of gold.
Step-by-step explanation:
The bracelet displaces the water by
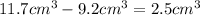 and thus has a volume of
and thus has a volume of
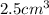 . If the bracelet has a mass of 45g, we know that the density of the bracelet is then
. If the bracelet has a mass of 45g, we know that the density of the bracelet is then
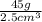 , which can be simplified to
, which can be simplified to
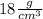 . This is closer to the density of gold, so it can be assumed that the bracelet is made of gold.
. This is closer to the density of gold, so it can be assumed that the bracelet is made of gold.