Answer:
Dalton's law of partial pressure
Step-by-step explanation:
In Dalton's law of partial pressure is states that the total pressure of a gas mixture is equal to the sum of the partial pressures of the component gasses.
This is the equation:
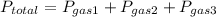 .. so on and so forth.
.. so on and so forth.
basically what Dalton is saying partial pressures are just fractions of the total pressure, think of it as if you cut 12 slices of pizza and 12 people grabbed a slice of the pizza. Each person has 1/12 of that pizza, 1/12 x 12 = 1.
let's say there is a container filled with nitrogen gas (N) , hydrogen gas (H), and ammonia gas (NH₃).
N= 40 atm
H=20 atm
NH₃= 10 atm
you would plug this information into this equation:
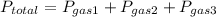
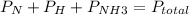
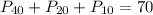
N is 40/70 of the gaseous mixture.
H is 20/70 of the gaseous mixture.
NH₃ is 10/70 of the gaseous mixture.
if you add these fractions up:
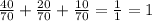
I really hope this helps a little!