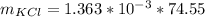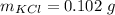Answer:
The mass is
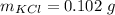
Step-by-step explanation:
From the question we are told that
The volume of oxygen produced is
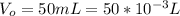
The temperature is
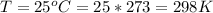
The pressure is
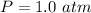
From the ideal gas law we have that
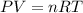
Where R is the gas constant with the value
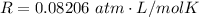
n is the number of moles making it the subject of the formula
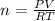
Substituting values
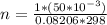

From the chemical equation
one mole of
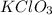 produces one mole of kCl and
produces one mole of kCl and
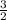 of oxygen
of oxygen
x mole of
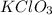 produces x mole of kCl and
produces x mole of kCl and
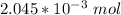 of oxygen
of oxygen
So
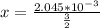
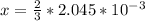

Now the molar mass of KCl is a constant with a value
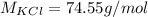
Now the mass of KCl is mathematically evaluated as
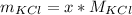
Substituting values