Answer:
0.025L of 2M HCl
Step-by-step explanation:
This is a dilution problem involving the equation
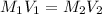
Where M is molarity in grams/liter and V is volume in liters, and 1 and 2 represent initial and final states, respectively.
We know that:
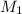 = 2, as 2M HCl is the starting substance
= 2, as 2M HCl is the starting substance
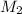 = 0.05, as 0.05M HCl is the desired final substance
= 0.05, as 0.05M HCl is the desired final substance
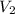 = 1, as 1 L is the desired volume of the final substance
= 1, as 1 L is the desired volume of the final substance
Now you can solve for
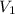 .
.
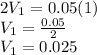
Therefore, 0.025L of 2M HCl is needed to make 1 L of 0.05M HCl