Answer:
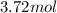
Step-by-step explanation:
Hello,
In this case, we consider that at STP conditions (273 K and 1 atm) we know that the volume of 1 mole of a gas is 22.4 L, thereby, for 83.4 L, the resulting moles are:
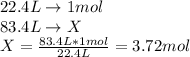
This is a case in which we apply the Avogadro's law which relates the volume and the moles as a directly proportional relationship.
Best regards.