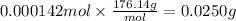Answer:
0.0250 g
Step-by-step explanation:
Step 1: Determine the molar mass of Vitamin C.
The molar mass is the mass in grams corresponding to 1 mole. In order to calculate the molar mass of vitamin C (C₆H₈O₆) we need to add the molar masses of the elements that compose it.
M(C₆H₈O₆) = 6 × M(C) + 8 × M(H) + 6 × M(O)
M(C₆H₈O₆) = 6 × 12.01 g/mol + 8 × 1.01 g/mol + 6 × 16.00 g/mol
M(C₆H₈O₆) = 176.14 g/mol
Step 2: Calculate the mass corresponding to 0.000142 mol of vitamin C.