Answer:
It will exert the pressure at a temperature of 153.44 K
Step-by-step explanation:
To answer this question, we shall be using the ideal gas equation;
PV = nRT
Since we are calculating the temperature, it can be made the subject of the formula.
Thus, this can be T = PV/nR
where P is the pressure = 900 torr
V is the volume = 0.75 L
n is the number of moles = 0.0705 mol
R is the molar gas constant = 62.4 L.Torr.
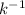 .
.
Plugging these values into the equation, we have;
T = (900 × 0.75)/(0.0705 × 62.4)
T = 153.44 K