Answer:
The answer is "
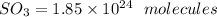 ".
".
Step-by-step explanation:
In the given question some information is missing, that chemical reaction, which can be described as follows:
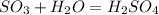
where,
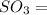 Sulfur trioxide
Sulfur trioxide
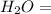 water
water
 = Sulfuric acid
= Sulfuric acid
calculating molecules of sulfur trioxide:
Formula:
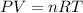
Where,
