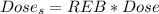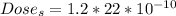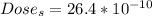Answer:
1
The mass of the Potassium-40 is
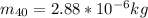
2
The Dose per year in Sieverts is
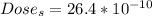
Step-by-step explanation:
From the question we are told that
The isotopes of potassium in the body are Potassium-39, Potassium-40, and Potassium- 41
Their abundance is 93.26%, 0.012% and 6.728%
The mass of potassium contained in human body is
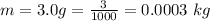 per kg of the body
per kg of the body
The mass of the first body is
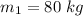
Now the mass of potassium in this body is mathematically evaluated as
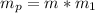
substituting value
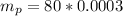
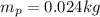
The amount of Potassium-40 present is mathematically evaluated as
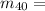 0.012% * 0.024
0.012% * 0.024
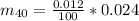
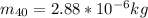
The dose of energy absorbed per year is mathematically represented as
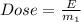
Where E is the energy absorbed which is given as
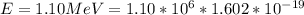
Substituting value
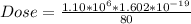

The Dose in Sieverts is evaluated as