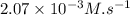Answer:
Initial rate of reaction is
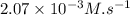 .
.
Step-by-step explanation:
It is a second order reaction.
Initial rate of reaction =
![k[O_(3)]_(0)[NO]_(0)](https://img.qammunity.org/2021/formulas/chemistry/college/j9hx7rhu5gurm67kil1sg4tjyhvnumxw62.png) , where k is rate constant,
, where k is rate constant,
![[O_(3)]_(0)](https://img.qammunity.org/2021/formulas/chemistry/college/8knqt7yctl0j02czdz1gyaaruv3sgcumg6.png) is the initial concentration of
is the initial concentration of
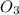 and
and
![[NO]_(0)](https://img.qammunity.org/2021/formulas/chemistry/college/bssowqx5wf8dtf1s8ozw3xs2v7rxhvx4y8.png) is the initial concentration of NO.
is the initial concentration of NO.
Here, k =
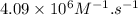 ,
,
![[O_(3)]_(0)=5.84* 10^(-6)M](https://img.qammunity.org/2021/formulas/chemistry/college/so4i8m67852uxwdhhj7wofhu1jwgok7omp.png) and
and
![[NO]_(0)=8.65* 10^(-5)M](https://img.qammunity.org/2021/formulas/chemistry/college/y5o3vvoxhwm4qorio2s36xtb43jdwqygme.png)
So, initial rate of reaction =
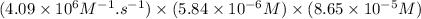
=
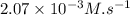
So, initial rate of reaction is