Answer:
The limiting reactant is Carbon dioxide,
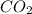
Step-by-step explanation:
The balanced reaction equation is:
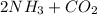 →
→
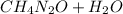
The mole ratio of ammonia to carbon dioxide is 2:1
142100/17g = 8358.8 mol of NH3
211400/44g = 4, 804.5 mole of CO2
Now:
4,804.5 mol of CO2 ×
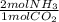 = 9,609 mol of NH3 present
= 9,609 mol of NH3 present
8,358.8 mole of NH3 ×
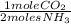 = 4,179.4 mol of CO2 present
= 4,179.4 mol of CO2 present
NH3 needs 8, 358.8 moles but had 9, 609 moles⇒ excess reactant
CO2 needs 4, 804,5 mol but had 4, 179.4 moles⇒ limiting reactant (used up completely)
The limiting reactant is carbon dioxide, CO2.