Answer:
Option "C" is the correct answer to the following question.
Step-by-step explanation:
Given:
Pressure in an automobile tire (P) = 1.88 atm
Temperature (K) = 25°C = 273 + 25 = 298 Kelvin
New temperature (K1) = 37°C = 273 + 37 = 310 Kelvin
Find:
New pressure in an automobile tire (P1) = ?
Computation:
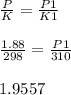
New pressure in an automobile tire (P1) = 1.9557
New pressure in an automobile tire (P1) = 1.96 (Approx)