Answer:
1.53 L.
Step-by-step explanation:
Given,
Volume pf hydrogen, V₁ = 153.48 mL = 0.15348 L
Temperature, T₁ = 14°C = 273+14 = 287 K
Pressure,P₁ = 973 kPa
1 atm = 101.325 kPa
973 kPa = 9.60 atm
At STP
P₂ = 1 atm
T₂ = 25° C = 273+25 = 298 K
V₂ = ?
Now,
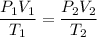
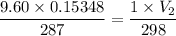
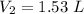
Hence, the volume at STP is 1.53 L.