Answer : The correct option is, 3.75 mole
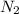
Explanation : Given,
Moles of
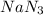 = 2.5 mol
= 2.5 mol
The balanced chemical equation is:
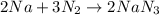
From the balanced reaction we conclude that
As, 2 moles of
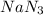 produce from 3 moles of
produce from 3 moles of
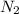
So, 2.5 moles of
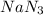 produce from
produce from
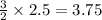 moles of
moles of
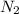
Therefore, the moles of
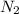 initially present was, 3.75 moles.
initially present was, 3.75 moles.