15.85 grams
Step-by-step explanation:
Here we want to get the number of grams present in 5 litres of chlorine gas at STP
At STP, 1 mole of gas occupies a volume 22.4 dm³
X moles will occupy 5L (litres)
Thus we have that :-
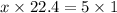
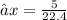
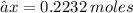
Mathematically, the number of grams is the product of the number of moles and molar mass.
Molar mass of chlorine gas is 71 g/mol
So,
Mass = Mole × Molar mass
→ 0.2232 × 71
→ 15.85 g