Answer:
The final temperature of the ice is
 .
.
Step-by-step explanation:
It is given that,
Energy, Q = 10 cal
Mass of ice, m = 2 g
Initial temperature,
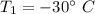
We need to find the final temperature of the ice. We know that the specific heat of ice is
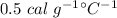
The heat added in terms of specific heat is given by :
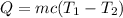
 final temperature of the ice
final temperature of the ice
c is specific heat of ice
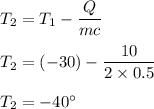
So, the final temperature of the ice is
 .
.