Answer: The pH of 0.000134 M solution of HCl is 3.873.
Step-by-step explanation:
When HCl dissociates in water then it will give hydrogen and chlorine ions. The reaction will be as follows.
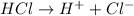
This means that 1 mole of HCl is giving 1 mole of hydrogen ions on dissociation.
Hence, pH of the given solution will be calculated as follows.
pH =
![-log [H^(+)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/hggt6ye9u4ukk181i3mah9cn5ybt9okq1d.png)
=
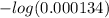
= 3.873
Thus, we can conclude that the pH of 0.000134 M solution of HCl is 3.873.