Answer:
0.215 L HCl
Step-by-step explanation:
Using the relationship (M1 x V1 = M2 x V2) between Molarity (M) and Volume (v or L), we can find the volume of the 2nd volume amount.
First we rearrange our relationship equation to:
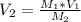
Now, plug in the values.
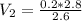
Calculating these values, we end up with the answer:
0.215 L HCl