Answer:
The volume of 1.0 AgNO₃ that would be required to precipitate 5.84 grams of NaCl is 99.93 ml
Step-by-step explanation:
Here we have the reaction of AgNO₃ and NaCl as follows;
AgNO₃(aq) + NaCl(aq)→ AgCl(s) + NaNO₃(aq)
Therefore, one mole of silver nitrate, AgNO₃, reacts with one mole of sodium chloride, NaCl, to produce one mole of silver choride, AgCl, and one mole of sodium nitrate, NaNO₃,
Therefore, since 5.84 grams of NaCl which is 58.44 g/mol, contains
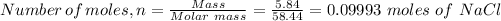
0.09993 moles of NaCl will react with 0.09993 moles of AgNO₃
Also, as 1.0 M solution of AgNO₃ contains 1 mole per 1 liter or 1000 mL, therefore, the volume of AgNO₃ that will contain 0.09993 moles is given as follows;
0.09993 × 1 Liter/mole= 0.09993 L = 99.93 mL
Therefore, the volume of 1.0 AgNO₃ that would be required to precipitate 5.84 grams of NaCl is 99.93 ml.