Answer:
P₂ ≈ 80 kPa
General Formulas and Concepts:
Math
Pre-Algebra
Order of Operations: BPEMDAS
- Brackets
- Parenthesis
- Exponents
- Multiplication
- Division
- Addition
- Subtraction
Algebra I
Chemistry
Unit 0
Temperature Conversion: K = °C + 273.15
Gas Laws
Gay-Lussac's Law:
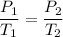
- P is pressure
- T is temperature (in Kelvins)
Step-by-step explanation:
Step 1: Define
P₁ = 87 kPa
T₁ = 50 °C
T₂ = 25 °C
P₂ = ?
Step 2: Identify Conversions
Kelvin Temp Conversion
Step 3: Convert
T₁ - 50 °C + 273.15 = 323.15 K
T₂ - 25 °C + 273.15 = 298.15 K
Step 4: Solve for P₂
- Substitute [GLL]:
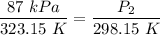
- Cross-multiply:
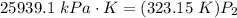
- Isolate P₂:
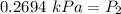
- Rewrite:
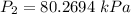
Step 5: Check
We are given 1 sig fig as our lowest. Follow sig fig rules and round.
80.2694 kPa ≈ 80 kPa