Answer: A.Greater than
Step-by-step explanation:
According to the neutralization law,

where,
 = molarity of stock solution = ?
= molarity of stock solution = ?
 = volume of stock solution = 100 ml
= volume of stock solution = 100 ml
 = molarity of dilute solution = 0.75 M
= molarity of dilute solution = 0.75 M
 = volume of dilute solution = 225 ml
= volume of dilute solution = 225 ml

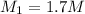
Thus Concentration of the original stock solution will be greater than that of the mixed solution.