Answer : The final volume of the sample of gas is, 120 mL
Explanation :
According to the Boyle's law, the pressure of the gas is inversely proportional to the volume of gas at constant temperature and moles of gas.
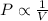
or,

where,
 = initial pressure = 1.5 atm
= initial pressure = 1.5 atm
 = final pressure = 5 atm
= final pressure = 5 atm
 = initial volume = 400 mL
= initial volume = 400 mL
 = final volume = ?
= final volume = ?
Now put all the given values in the above formula, we get:
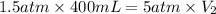
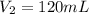
Therefore, the final volume of the sample of gas is, 120 mL