Answer:
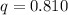 Kj/mol
Kj/mol
Its an endothermic reaction
Step-by-step explanation:
Enthalpy can be evaluated by using following formula -

where
q is the enthalpy or change in internal energy
m is the mass in kg
c is the specific heat
and
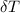 is the change in temperature
is the change in temperature
Mass of HCl is equal to
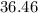 g/mol
g/mol
or
Mass of HCl is equal to
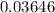 Kg/mol
Kg/mol
Substituting the given values in above equation, we get-
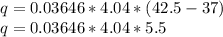
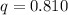 Kj/mol
Kj/mol
Enthalpy is positive thus it is an endothermic reaction