Answer:
See Below:
Step-by-step explanation:
Part A
Nickel(II) hydroxide: in this compound, Nickel has oxidation state of 2+, and we can safely assume hydroxide ion OH- has -1 oxidation. Hence, our chemical formula would be:
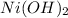
Part B
Acids give off hydrogen ion as the only positively charged ions in solution. Since oxidation number of hydrogen is 1+, chemical formula of carbonic acid is :
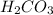
Part C
- Nitric =
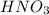
- Nitrous acid =
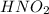
- Chloric acid =
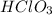
- Hydrochloric acid = HCl