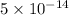Answer : The hydroxide ion concentration of a solution is,
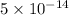
Explanation :
As we know that
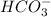 dissociates in water to give hydrogen ion
dissociates in water to give hydrogen ion
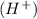 and carbonate ion
and carbonate ion
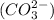 .
.
As, 1 mole of
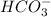 dissociates to give 1 mole of hydrogen ion
dissociates to give 1 mole of hydrogen ion
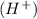
Or, 1 M of
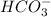 dissociates to give 1 M of hydrogen ion
dissociates to give 1 M of hydrogen ion
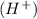
So, 0.200 M of
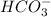 dissociates to give 0.200 M of hydrogen ion
dissociates to give 0.200 M of hydrogen ion
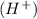
Now we have to calculate the hydroxide ion concentration.
As we know that:
![[H^+][OH^-]=1* 10^(-14)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/lwwg2aqsf2bqkh60ypo3cmst5xsy4rljb4.png)
![0.200* [OH^-]=1* 10^(-14)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/94rb3r9u4lmqyuh3r8t7xox2gl7jzr1hqh.png)
![[OH^-]=5* 10^(-14)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/e9xspqgee43vf2xo6nk6oulemxr8f7umfs.png)
Therefore, the hydroxide ion concentration of a solution is,