Answer:
3.071*10^{-6}V
Step-by-step explanation:
To find the potential difference you take into account the kinetic energy of the electron generated by the potential:
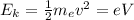 (1)
(1)
m: mass of the electron = 9.1*10^{-31}kg
v: velocity of electron
V: potential difference
e: charge of electron = 1.6*10^{-19}C
Thus, is necessary to find the velocity. By using the Broglie's relation you obtain:
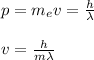
h: Planck's constant = 6.62*10^{-34}Js
wavelength = 700*10^{-9}m
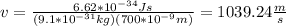
By doing V the subject of the formula (1) you obtain:
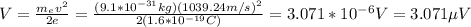
the potential difference required is 3.071*10^{-6}V