Answer: True
Step-by-step explanation:
Weak electrolytes are those solutions which do not undergo complete dissociation when dissolved in water. The dissociation of weak electrolytes is given by an equilibrium.
Example:
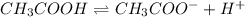
Strong electrolytes are those solutions which undergo complete dissociation when dissolved in water. The dissociation of strong electrolytes is given by a right arrow.
Example:
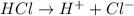
Thus the degree to which various compounds will dissociate in solution varies greatly is true.