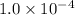The question is incomplete, here is the complete question:
Iron (III) oxide and hydrogen react to form iron and water, like this:
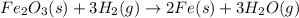
At a certain temperature, a chemist finds that a 8.9 L reaction vessel containing a mixture of iron(III) oxide, hydrogen, Iron, and water at equilibrium has the following composition.
Compound Amount
Fe₂O₃ 3.95 g
H₂ 4.77 g
Fe 4.38 g
H₂O 2.00 g
Calculate the value of the equilibrium constant Kc for this reaction. Round your answer to 2 significant digits.
Answer: The value of equilibrium constant for given equation is
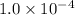
Step-by-step explanation:
To calculate the molarity of solution, we use the equation:
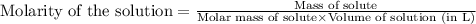
Given mass of hydrogen gas = 4.77 g
Molar mass of hydrogen gas = 2 g/mol
Volume of the solution = 8.9 L
Putting values in above expression, we get:
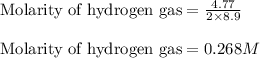
Given mass of water = 2.00 g
Molar mass of water = 18 g/mol
Volume of the solution = 8.9 L
Putting values in above expression, we get:
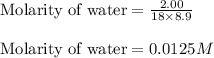
For the given chemical equation:
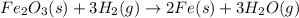
The expression of equilibrium constant for above equation follows:
![K_(eq)=([H_2O]^3)/([H_2]^3)](https://img.qammunity.org/2021/formulas/chemistry/college/3rxxjwerno7iddz5htucm2pqp3mtnzqynv.png)
Concentration of pure solids and pure liquids are taken as 1 in equilibrium constant expression.
Putting values in above expression, we get:
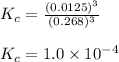
Hence, the value of equilibrium constant for given equation is