Answer: The partial pressure of hydrogen gas is 0.503 atm and that of oxygen gas is 0.248 atm
Step-by-step explanation:
We are given:
Moles of hydrogen gas = 6.7 moles
Moles of oxygen gas = 3.3 moles
Mole fraction of a substance is given by:
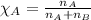
Mole fraction of hydrogen gas,
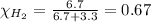
Mole fraction of oxygen gas,
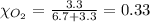
To calculate the total pressure of the container, we use the equation given by Raoult's law, which is:
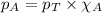
where,
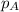 = partial pressure of substance
= partial pressure of substance
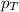 = total pressure
= total pressure
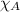 = mole fraction of substance
= mole fraction of substance
We are given:
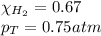
Putting values in above equation, we get:
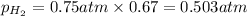
We are given:
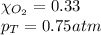
Putting values in above equation, we get:
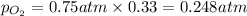
Hence, the partial pressure of hydrogen gas is 0.503 atm and that of oxygen gas is 0.248 atm