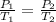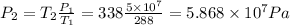Answer:
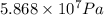
Step-by-step explanation:
If half the gas is drawn then pressure would have dropped by half
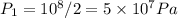
Assuming ideal gas, if temperature rises from 15c (T1 = 15 + 273 = 288 K) to 65 c (T2 = 65 + 273 = 338 K), then we have the following equation for ideal gas