Answer:
The longest wavelength of radiation is 241nm and it lies in ultraviolet region.
Step-by-step explanation:
The minimum energy required to break o-o bond is 495kJ/mole.
The photon does not have mass and the energy of the single photon depends entirely on the wavelength and is given by
e =hc/λ
where, h is the Planck constant,
c is the speed of light
e is the energy of photon
λ is the wavelength
From the Planck formula we can understand that energy of the photon is quantized.
E = e.Nₐ
e = E/Nₐ =
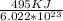 = 8.22*10⁻¹⁹ J/photon
= 8.22*10⁻¹⁹ J/photon
λ = hc/E=
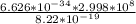
λ = 2.41*10⁻⁷ m = 241 nm
The longest wavelength of radiation is 241nm and it lies in ultraviolet region.