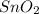Answer: The empirical formula for the given compound is
Step-by-step explanation:
Mass of Sn = 11.775 g
Mass of O = 3.180 g
To formulate the empirical formula, we need to follow some steps:
Step 1: Converting the given masses into moles.
Moles of Sn =
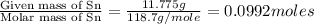
Moles of O =
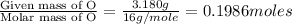
Step 2: Calculating the mole ratio of the given elements.
For the mole ratio, we divide each value of the moles by the smallest number of moles calculated which is 0.0992 moles.
For Sn =
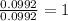
For O =
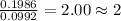
Step 3: Taking the mole ratio as their subscripts.
The ratio of Sn : O = 1 : 2
Hence, the empirical formula for the given compound is