Answer:
There are 2.01 × 10²³ atoms in the substance.
General Formulas and Concepts:
Stoichiometry
- Dimensional Analysis
- Molar Ratio
- Avogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.
Step-by-step explanation:
Step 1: Define
Identify given.
Initial amount of 480 grams of substance.
Molar Weight (Mass) of substance: 1440 g
Step 2: Convert
- [DA] Set up:
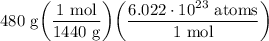
- Evaluate:
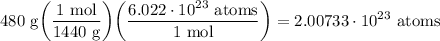
- Round [Sig Figs]:
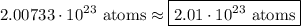
∴ we have converted the given amount of substance into amount of atoms.
---
Topic: AP Chemistry
Unit: Stoichiometry