Complete Question
The diagram for this question is shown on the first uploaded image
The question to solve is shown on the second uploaded image
Answer:
- The reaction is exothermic
- The temperature of the water bath goes up
- The piston move out
- The system release energy
- The amount of energy released is
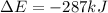
Step-by-step explanation:
From the question we are told that
The pressure is
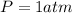
The amount of heat flow out of the system is
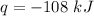
The workdone by the system is
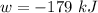
The first question is to state whether the reaction is exothermic
Yes this reaction is exothermic because heat is being transferred out of the system
The second second question is to determine whether the temperature of the water goes up or down
The temperature of the water goes up because above we see that the reaction is exothermic which means that heat is being transferred from the system to the surrounding which i the water bath in this case so this then implies that the temperature of the water in the water bath would go up
The third question is to determine whether the piston moves in or out
Now since work is done by the system which would mean that its pressure would increase but we require a constant pressure of 1 atm and to achieve this the there is need for an increase in volume in order to return the pressure back to 1 atm and this can be achieved by the piston moving out
The fourth question is to determine whether this reaction releases or absorbs energy
We can determine this by looking at the first law of thermodynamics
which is stated mathematically as
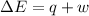
Where w is the workdone by the system given in the question as (-179 kJ)
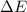 is the internal energy
is the internal energy
q is the heat flowing out of the system which is given as (-108 kJ)
Substituting this into the question we have that
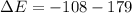
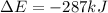
This negative internal energy indicates that energy is been released from the system.
The fifth ask that we determine how much energy is been absorbed or released by the system
The solution is already obtained in the fourth question an it is
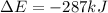
When to understand that heat flow into a system mean that the system
gains energy in form of heat so that energy with respect to the system is positive
Also When heat i transferred out of a system it means that the system losses energy in form of heat so that energy in term of the system is negative
Note also that when an external body does work on a system that the workdone is done is positive
But when a system does work on an external body the workdone is negative
In the case of Internal energy
When energy is absorbed by a system the then that energy in terms of the system is positive
But when energy is released by the system to the surrounding then that energy is negative with respect to the system