Answer:
4.62 kJ of heat is given off in the reaction.
Step-by-step explanation:
Let's assume specific heat of calorimeter is negligible.
So, amount of heat given off, q = heat absorbed by solution
= (
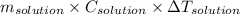 )
)
where, m is mass, C is specific heat and
 is change in temperature.
is change in temperature.
So, q =
![[120.0g* 4.184\frac{J}{g.^(0)\textrm{C}}* (29.20-20.00)^(0)\textrm{C}]](https://img.qammunity.org/2021/formulas/chemistry/high-school/rzi6ziye5tud0cymteln0ye4wm1mnniotn.png)
= 4619 J
= 4.62 kJ (3 sig. fig.)
Option (A) is correct