Answer:
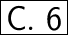
Step-by-step explanation:
Orbitals:
- Wave function that describes the location, electronic cloud and wave-like properties of an electron is called an orbital.
Types:
There are 4 types of main orbitals.
- s orbital (can accommodate 2 electrons)
- p orbital (can accommodate 6 electrons)
- d orbital (can accommodate 10 electrons)
- f orbital (can accommodate 14 electrons)
![\rule[225]{225}{2}](https://img.qammunity.org/qa-images/2023/formulas/biology/high-school/vdifidejf5i8c49g0hg7.png)