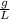Answer:
The density of O₂ gas is 1.71
Step-by-step explanation:
Density is a quantity that allows you to measure the amount of mass in a given volume of a substance. So density is defined as the quotient between the mass of a body and the volume it occupies:
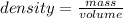
An ideal gas is characterized by three state variables: absolute pressure (P), volume (V), and absolute temperature (T). The relationship between them constitutes the ideal gas law, an equation that relates the three variables if the amount of substance, number of moles n, remains constant and where R is the molar constant of the gases:
P * V = n * R * T
So, you can get:
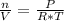
The relationship between number of moles and mass is:
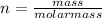
Replacing:
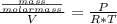
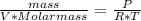
So:
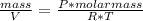
Knowing that 1 mol of O has 16 g, the molar mass of O₂ gas is 32
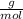 .
.
Then:
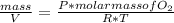
In this case you know:
- P=1.27 atm
- molar mass of O₂= 32
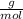 .
.
- R= 0.0821
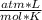
- T= 16 °C= 289 °K (0°C= 273°K)
Replacing:
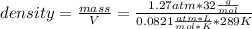
Solving:
density= 1.71
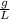
The density of O₂ gas is 1.71