The percent yield of the reaction is 41.99 % If 1.0 mol of Mg is mixed with 2.0 mol of Br2, and 0.42 mol of MgBr2 is obtained.
Step-by-step explanation:
Data given:
number of moles of magnesium = 1 mole
number of moles of bromine gas = 2 moles
moles of magnesium bromide obtained = 0.42 moles
percent yield =?
balance chemical reaction:
Mg +
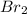 ⇒ Mg
⇒ Mg
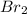
1 mole of Mg reacted to give 1 mole of Mg
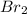
1 mole of Br2 reacted to give 1 moles of Mg
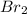
2 moles of Br2 reacted to give 2 moles of Mg
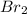
limiting reagent is Mg
so, mass obtained by 1 mole Mg = 1 x 184.11
mass obtained = 184.11 (theoretical yield)
actual yield = 0.42 x 184.11
actual yield = 77.32 grams
percent yield =
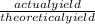 x 100
x 100
putting the values in the equation:
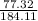 x100
x100
percent yield = 41.99 %