7.88 grams is the mass of oxygen gas results from the decomposition of 17.00 g of calcium chlorate.
Step-by-step explanation:
Data given:
mass of calcium chlorate = 17 grams
atomic mass of calcium chlorate = 206.98 grams/mole
mass of oxygen formed =?
atomic mass of oxygen gas = 32 grams/mole
balance chemical reaction:
Ca(Cl
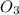 )
)
 ⇒
⇒
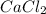 + 3
+ 3

Number of moles =

putting the values in the equation:
number of moles =
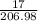
number of moles of calcium chlorate = 0.0821 moles
1 mole of calcium chlorate decomposes to release 3 mole of oxygen
0.0821 moles will release x moles of oxygen gas
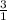 =
=
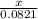
x = 0.246 moles
mass = atomic mass x number of moles
mass = 0.246 x 32
mass = 7.88
7.88 grams of oxygen produced from decomposition of 17 grams calcium chlorate