Answer:
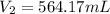
Step-by-step explanation:
Hello,
In this case,
One uses the Boyle's law that allows us to understand the pressure-volume behavior at constant temperature as an inversely proportional relationship:

Thus, solving for the volume at the new 88.2 kPa pressure, one obtains:
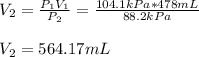
Best regards.