132.67 K is the temperature at which 55 grams of chlorine gas exert a pressure of 245 kPa at a volume of 3.4 liter.
Step-by-step explanation:
Data given:
mass of chlorine gas = 55 grams
atomic mass of chlorine gas = 71 grams/mole
pressure of chlorine gas = 245 kPa 0R 2.41 atm
volume of the chlorine gas = 3.4 litres
temperature of the chlorine gas = ?
R = 0.08201 Latm/moles K
n =?
Assuming that chlorine behaved like ideal gas, the formula used will be,
PV = nRT
number of moles =

number of moles=
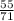
number of moles = 0.77
putting the values in ideal gas equation:
T =
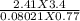
T = 132.67 K
temperature is 132.67 K