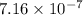Answer: The concentration of hydroxide ions at this temperature is
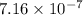
Step-by-step explanation:
When an expression is formed by taking the product of concentration of ions raised to the power of their stoichiometric coefficients in the solution of a salt is known as ionic product.
The ionic product for water is written as:
![K_w=[H^+][OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/7jdjcuyzjoh0xkeaez8b0dl2066xe1tebt.png)
![5.13* 10^(-13)=[H^+][OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/j2nck3qprflacp8woxacda1igvvbl05m68.png)
As
![[H^+]=[OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/lp8fbaeok2xwhex5vwkyo0d1wjhj7t32mz.png)
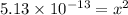
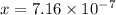
The concentration of hydroxide ions at this temperature is