The molarity of the given solution is 0.1 M.
Step-by-step explanation:
We have to find the molarity of calcium chloride by converting the mass of CaCl₂ into its moles and dividing it by its volume in Litres.
We have to find the moles by dividing the mass of the substance by its molar mass.
Moles of CaCl₂ =
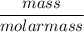
=
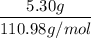
= 0.05 moles
Molarity =
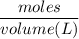
=
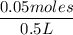
= 0.1 M
So the molarity of the given solution is 0.1 M.