193.38 K was the initial temperature of the krypton.
Step-by-step explanation:
Data given:
Initial volume of the krypton gas = 6 litres
initial pressure of the krypton gas = 0.960 atm
initial temperature of the krypton gas = ?
final volume of the krypton gas = 7.70 litres
final pressure of the Krypton gas = 1.25 atm
final temperature of the krypton gas = 55 degrees or 273.25+55 = 323.15 K
Applying the Combined Gas Laws:
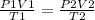
Rearranging the equation:
T1 =
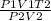
Putting the value in the equation:
T1 =
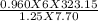
T1 = 193.38 K
Initial temperature of the krypton gas is 193.78 K