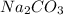Step-by-step explanation:
the percentage can be also written as grams.
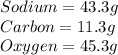
Using their atomic mass, convert from grams to mol.
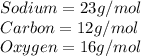
Convert.
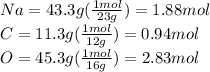
Now find the lowest number of moles and divide each element by it. In this case, the lowest number is 0.94mol
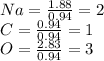
So, your empirical formula would be: