Answer:
pH = 5.80
Step-by-step explanation:
The buffer solution is:
H₂CO₃(aq) + H₂O(l) ⇄ HCO₃⁻Na⁺(aq) + H₃O⁺(aq)
To find the pH of the buffer solution we will use the Henderson-Hasselbalch equation:
![pH = pKa + log(([NaHCO_(3)])/([H_(2)CO_(3)]))](https://img.qammunity.org/2021/formulas/chemistry/college/ipe4j3ns74cfqmdfa26fk0eadkmi3kdapn.png) (1)
(1)
First, we need to find the concentration of the buffer solution. For the NaHCO₃ we have:
![[NaHCO_(3)] = (mol)/(V) = (m)/(M*V)](https://img.qammunity.org/2021/formulas/chemistry/college/vjg44i5be7rc96zyzzdxta121gwbzlc6lm.png)
Where:
m: is the mass of the NaHCO₃ = 7.20 g
M: is the molar mass of the NaHCO₃ = 84.007 g/mol
V: is the volume of the solution = 400.0 mL
Hence, the concentration of NaHCO₃ is:
![[NaHCO_(3)] = (7.20 g)/(84.007 g/mol*400.0 \cdot 10^(-3) L) = 0.214 M](https://img.qammunity.org/2021/formulas/chemistry/college/tmlmk5wseu82uw0qjdot68x4suewe160fa.png)
Now, the concentration of H₂CO₃ is:
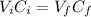
Where:
Vi: is the initial volume of H₂CO₃ = 56.0 mL
Ci: is the initial concentration of H₂CO₃ = 5.60 M
Vf: is the final volume of H₂CO₃ = 400.0 mL
Cf: is the final concentration of H₂CO₃ (to find)
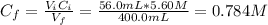
Finally, we can use the equation (1) to find the pH of the buffer solution:
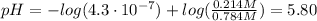
I hope it helps you!