This is an incomplete question, here is a complete question.
Determine the specific heat of a material if a 35 g sample absorbed 96 J as it was heated from 293 K to 313 K.
Answer : The specific heat of a material is, 0.137 J/g.K
Explanation :
Formula used to calculate the specific heat of a material is:
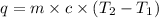
where,
q = heat absorbed = 96 J
m = mass of sample = 35 g
c = specific heat capacity of material = ?
 = initial temperature = 293 K
= initial temperature = 293 K
 = final temperature = 313 K
= final temperature = 313 K
Now put all the given values in the above formula, we get:
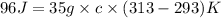
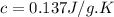
Therefore, the specific heat of a material is, 0.137 J/g.K