Answer:
The new pressure of the gas is 42 pounds per square inches.
Explanation:
When the temperature remains constant, the volume of a gas is inversely proportional to the pressure of the gas.
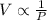
We can rewrite the above equation is
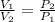 [at constant temperature]
[at constant temperature]
At a pressure of 14 pounds per square inches, a balloon is filled with 93 cubic inches of gas.
The volume of the gas is decreased to 31 cubic inches.
Here,
 = 93 cubic inches,
= 93 cubic inches,
 = 14 pounds per square inches,
= 14 pounds per square inches,
 =31 cubic inches,
=31 cubic inches,
 = ?
= ?
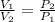
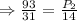
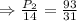
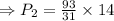
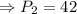 pounds per square inches
pounds per square inches
The new pressure of the gas is 42 pounds per square inches.